Vector Insects
Green Peach and Potato Aphids
Several different aphids can be found in potato fields. Aphids of themselves can cause wilting damage by sucking out nutrients from foliage and stem tissues and this may be a problem especially in nursery crops. But, the real problem caused by aphids in commercial fields is their ability to carry pathogenic viruses. In potato fields, the common viruses are potato leaf roll virus (PLRV), the mosaic viruses (e.g., PVA, PVY) and alfalfa mosaic virus (AMV, “calico virus”). Persistence is the key to the spread of a virus by aphids in the potato field. For this reason, the most important of the aphids in potato is the Green Peach Aphid (GPA) (Myzus persicae) which will persistently carry PLRV, usually the major viral problem on potato. Other aphids that will carry viruses non-persistently are Potato Aphids which may carry PLRV and others as the GPA, and Alfalfa Aphid which carries the non-persistent AMV/calico.
Description
GPA undergo three stages of development: adult, nymph and egg.
Adults have a tear-drop shape. Winged adults are bright green with a dark head and thorax, and a greenish abdomen with dark patches. They secrete a sticky substance called honeydew. In contrast, potato aphids are larger with more elongated bodies. The best method to obtain a positive identification is to view under a microscope at a 10X magnification and observe the head. Look at the shape and length of the converging tubercules or projections at the base of the antennae as viewed from above.
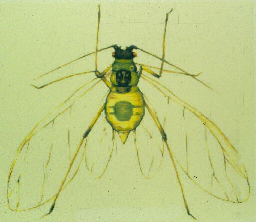

Nymphs are actually wingless female adults. They have a tear-drop shape and are about 1/10 inch long. During the early part of the season, they are light yellowish green to pinkish, and later in the season they are pink to pale orange. Nymphs move around and usually colonize on lower leaves.
[See diagrams comparing GPA with the potato aphid adults and nymphs courtesy of McCain Foods.]
Eggs are small and oval, and are shiny black.
Life Cycle
Eggs are the overwintering form of GPA. They overwinter in trees of the Prunus species such as wild plum, chokecherry, apricot. These hosts have been shown to have different preferences for GPA in different parts of the country. Harsh winters may kill some of the eggs and conversely more eggs may survive mild winters. The eggs survive in the bark of axillary buds. Hatching occurs in the spring just after full bloom (“green tip” stage) and nymphs, wingless female adults, emerge feeding for two or three generations on the leaves of the tree. In late spring, winged adults are produced and migrate to secondary hosts such as potato. But they are more likely to move to one of over 100 species of plants that can serve as secondary hosts. Some of the more common of these hosts include mustards, nightshades, ground cherries, and several ornamental or vegetable crops. During spring migration, winged adults travel many miles to many hosts depositing nymphs in colonies up to 20 on the lower third of plants. Later these nymph colonies will produce more winged adults that travel further (summer migration). During this migration cycle is when viral transmission occurs as the nymphs pick up virus from the colonized plant and the winged adults then transmit them to other plants. Many generations can be produced; the turn around rate reported is every eight to 14 days at 70oF. With the exception of the overwintering generation, the females give live birth to only females so their reproductive energy is very streamlined. With each generation, a greater proportion of winged adults, both male and female, are produced, and more are flying and spreading viruses. Toward the end of the season, the winged adults migrate to Prunus trees to lay the eggs that will overwinter.
Although the normal life cycle of the GPA involves overwintering in trees, GPA are also able to remain active all year round, and thereby overwintering, in greenhouses on bedding plants. An Idaho survey conducted in 1990 showed that over a third of the salable bedding plants were infested with GPA. These would be capable of escape either from the greenhouse or from home gardens developed with aphid-infested plants into potato fields.
Damage

GPA nymphs ingest sap from leaf veins and, if sufficiently severe, could cause some wilting damage. However, in potato fields, this is not why GPA is a concern to production. The concern dealing with all aphids that feed on potato is that they are vectors of pathogenic viruses, that is, they can carry viruses and inject them into healthy plants. The main concern with GPA over other aphids is that it can carry the PLRV persistently. Potato Aphid, for example, does not transmit PLRV persistently (non-persistent vector). Non-persistence means that the potato aphid picks up the PLRV, for instance, and injects all that if picked up on the next plants it feeds on. After that it must re-infect itself by feeding on another infected plant before it can spread the virus to another plant. GPA, on the other hand being a persistent vector, need only to pick up the viruses once and then it can infect one plant after the other without ever landing on another infected plant again.
Potato Leaf Roll (PLRV)
Note that viral infection and symptoms also occur in plants arising from seed tubers that are infected with the virus. See Biological Management -- Seed Certification.
Vine — Early season symptoms develop mostly on young leaves. They appear upright, pale and rolled. Leaves are crisp and stiff, and give a rattling noise when shaken. Plants are usually stunted and rigid when the seed tuber or piece was infected. Later in the season, leaf rolling is increased and all leaflets are affected. The leaf rolling should be confused with wilts such as Verticillium wilt (early dying) with which leaflets are limp and browning, and one side of the plant is initially affected. Black leg will also show a leaf roll but always look for the infected stem at the base. Plants invaded with Euro. corn borer will also leaf roll so look an entry hole of the larva.
Tuber — PLRV can affect tuber quality. This symptom is called net necrosis and appears as translucent spots from the tuber center extending ray-like. This is a concern because when fried these spots will turn brown resulting in polka-dotted french fries and potato chips. Note that this symptom can also be confused with freezing damage (see physiological disorders).
PLRV Transmission
Several species of aphids can transmit PLRV, but the GPA is by far the most efficient vector of this virus. PLRV transmission is more complicated compared to other potato viruses. PLRV is concentrated in the phloem of the plant. To pick up this virus an aphid must feed from the phloem which occurs only if the plant is a host plant. When the aphid ingests the virus, the virus will pass through the wall of the gut into the blood of the aphid. The virus will eventually move to the salivary gland where it can potentially be transmitted to the next plant the aphid injects saliva into. The virus can be picked up in less than 30 minutes of feeding, but there is a lag time of 12 to 36 hours before the virus will enter the salivary gland and be able to be passed on to the next plant. A major aspect of this type of transmission is that it is persistent and the aphid will be able to transmit the virus for the rest of its life.
Note: PLRV and other potato viruses are NOT passed from aphid to aphid. Each nymph must obtain the virus from an infected plant. These viruses also are NOT passed on from generation to generation. Each spring, the nymphs must pick up new virus fresh from emerged hosts which include many weeds such as nightshades, plants growing from infected seed tubers, plants growing in cull piles from infected discarded tubers, or from infected volunteer potatoes growing in the field of last year’s crop.
Monitoring
The inability to predict the occurrence of GPA populations causes difficulty in aphid and viral management. Several sampling tools have been used to aid in predicting GPA populations in potato. Large suction traps and smaller yellow pan traps have been used. However, current evidence indicates that these may not work best. Recommendations for GPA population monitoring rely on regular leaf sampling for aphid presence. Regular monitoring for GPA will allow the grower to identify the presence of the aphid at the earliest possible time. Beginning early in the season, monitor nearby Prunus trees and weed hosts such as mustards, nightshades and ground cherry. GPA infestations tend to begin or be higher near the edges of fields, particularly the windward side of the field and near building and shelter belts; special monitoring of these areas may provide warning on the early presence of aphids. Home gardeners and truck farmers should also be monitoring, not only their potato plants but also other garden plants and weeds in the area.
As soon as full leaves are out on the potato plant, sampling should be initiated in seed fields. GPA are more likely to be found on the lower third of the plant, so leaves should be chosen from this area of the plant. The entire field should be covered with a cris-cross or diagonal pattern when sampling. It is important to properly identify them so it’s best to collect them for later identification under magnification and note the form of the aphids, winged or wingless. The form of the aphids indicates the status of the aphid population in the field. If wingless aphids are present, colonies are likely to have been established in the field. However, if only winged aphids are present, the aphids found likely arrived only recently into the field. A minimum of 50 leaves should be sampled from across a field, but a 100-leaf sample will give an even better estimate of their potential presence. Monitoring for the aphids should continue as long as the vines are green and growing.
Leaf-sample thresholds vary a great deal depending on the type of potato being grown. For seed potato, a threshold of 10 wingless GPA per 100 leaves has been established in the upper Midwest. This threshold is used to eliminate treatments that are of little value. Some other seed producing areas use a zero-tolerance threshold. Thresholds to reduce net necrosis vary between different production regions. Idaho indicates that their threshold is 10 wingless aphids per 50 leaves, while the upper Midwest (MN, ND, WI) threshold is set at 30 wingless aphids per 100 leaves.
Management
Potato Seed Certification — GPA and other aphids are not a major problem as long as they do not pick up potato viruses be they PLRV or mosaic viruses. A major source, though not the only source, of these pathogens is infected seed tubers that are planted. Keeping the viral load to a minimum is achieved primarily through testing of seed tubers and growing those which were found to be viral free. This job is done through the States’ Potato Certification Associations. Viral levels in nuclear and early generations of certified seed are low enough to prevent problems from developing if aphid populations are managed properly. Therefore, it is crucial to plant certified seed. This is also true home gardeners and small acreage growers.
Biological — Several other cultural practices can be undertaken to reduce the potential for developing PLRV and other viruses. The virus pool can be reduced by eliminating cull piles and volunteer potatoes growing in the previous years’ potato fields. If possible, seed potato producing areas should be isolated from production areas. Weeds such as mustards and nightshades that may host GPA in the spring should be controlled in and around potato fields. Roguing of infected plants can help to reduce the level of viruses in the field. Proper roguing techniques should include the removal of the infected plant, plus the adjacent plants on all four sides of the infected plant. Some predation of nymphs (wingless females) by beneficial insects is possible especially during mid season. Until recent uninformed political pressure, genetically-modified (GMO) potato were being developed that were immune to infection by PLRV by not allowing the virus to replicate itself through disruption of its protein coat. Hopefully, this politicized climate will change as more people become better informed.
Varietal Sensitivity
|
(sources: "North American Potato Varieties" PAA, "Characteristics of Potato Varieties" PNW #454, "San Luis Valley Production Manual" XCM-181.)
Chemical — As with all vectored viruses, PLRV is managed by managing the vector along with minimizing the viral pool. If possible, nearby Prunus trees that may harbor overwintering eggs and be the first hosts in the spring should be treated for GPA. Optimizing GPA management requires regular monitoring, proper insecticide timing and use of the most effective insecticides. So, a major management tool for PLRV is insecticidal control of the GPA. Usually some type of systemic, broad-spectrum, soil-applied product is applied at planting protecting plants against several insects including GPA, Colorado potato beetles and potato psyllids. Recent products containing imidacloprid or thiamethoxam last up to 13 weeks after planting. Phorate, a commonly used inexpensive product, gives sporadic control of GPA and offers up to eight weeks of protection. Later season foliar treatments have been largely relied upon in the past to control GPA. Without doubt, Monitor is the best GPA control product currently registered but shows poor control of Colorado potato beetle. Multiple applications of Monitor in a year are to be avoided so selection pressure on the aphid population can be lessened and resistance to the product developed by GPA. If additional treatments are needed, Thiodan and Provado are rated as good products for both GPA and Col. potato beetle. Provado should not be used on fields where Admire was used at planting since they are both imidacloprid. Pyrethroids in general along with Sevin and methyl parathion are listed as products that will likely encourage the development of severe GPA problems. There are other products that are labeled for potato aphid but NOT for green peach aphid; they also may not very effective against either.
Resistance
A very important consideration for insecticidal control of GPA is to avoid the development of aphid resistance which has developed in several States on the East Coast and in the Great Lakes region. GPA is one of a few species of insects like the Colorado potato beetle that has developed resistance to all major insecticide classes. In some areas such as Nebraska, resistance by GPA or Col. potato beetle has not appeared yet. To delay or prevent resistance developments, it is crucial to keep the number of application of all types of insecticides to a minimum, to rotate between insecticide types within the year, and to apply products that will affect more than one pest with application. The latter requires careful monitoring and application timing as well as choosing the right product. Treatments should be chosen to minimize selection pressure.
Quick Review
Appearance:
- Adult (winged) — bright green with dark head and thorax, and dark patches on abdomen; tear-drop body shape; 1/10 inch long
- Nymph (wingless adult) — light yellowish green to pinkish to pale orange; like adult but wingless
- Egg — small, black and oval, found only on Prunus trees
Life Cycle:
- Overwinters as egg in Prunus trees
- Many generations during the season starting in April/May
Damage:
- Aphid — possible slight wilting
- Carries viruses especially potato leaf roll (PLRV)
- PLRV - stunting, leaf roll, tuber yield and quality loss
Management:
- Plant certified seed
- Locate away from Prunus trees
- Control/treat spring hosts including trees and weeds
- Eliminate cull piles and volunteer potatoes
- Beneficial insects
- Monitor in field, on bedding plants and in gardens
- Roguing
- Systemic soil and foliar insecticides
- GMO potato engineered with reverse viral coat protein
Sources:
GPA - Prunus trees, bedding plants, migration, gardens, nurseries
PLRV - seed tubers, weed hosts, cull piles, volunteer plants, bedding plants, gardens, nurseries
Potato Psyllids
Outbreaks of potato psyllids (Paratrioza cockerelli) are very sporadic but have occurred suddenly and over vast areas. Psyllids are most found from the Rio Grande River valley and north in the High Plains States. They are very small insects, the adults are about the size of the head of a pin while the damaging nymph is closer in size to the point of a pin. The damage to plants is due to the injection of toxin produced by the insect into the plant during its feeding.
Description
Potato psyllids go through three stages in their development - adult, egg and nymph. The nymph is the cause of problems on the plant.
Adults, nicknamed “jumping plant lice,” are not damaging. They look like a tiny cicada for those familiar with this noisy insect and will jump and fly when slightly disturbed. Adults are about a tenth of an inch long with wings held roof-like over the body. Initially light green for their first three days, they turn black with white markings, usually stripes. Their eyes are bulging or prominent and their legs are well developed.
Eggs are orange-yellow and appear at the end of short stalks, similar to but much smaller than the white eggs of lacewings (not a common predator). Eggs appear in clumps and usually, but not exclusively, on the underside of apical leaves.
Nymphs are the damaging form of this insect. They are tiny (pin-head) and flat. Their most notable appearance is that they look like overlapping scales (reminding me of a tiny trilobite of the Paleozoic Era). This scale-like appearance is ringed with tiny hairs. Young nymphs are pale brown and turning pale green with successive molting. There are four (five instars) molts until adult metamorphosis. Nymphs are mostly found in the upper canopy and on the underside of leaves. Nymphs are quiescent and, unlike aphid nymphs, do not move around much. Nymphs excrete a waxy salt-like or sugar-like white granular substance. They are sucking-type insects, but their damage is NOT due to feeding but due to the secretion of a toxin into the plant with their saliva. This toxin affects growth of all the plant’s parts and causes a symptom called “psyllid yellows.” Toxin occurrence in nymphs is irregular as it seems to an inherited trait. So the presence of psyllid nymphs does not necessarily mean that psyllid yellows will occur. Potato psyllids do NOT seem to be vectors of haywire, potato leaf roll nor aster yellow.


Life Cycle
Adults overwinter along the Rio Grande River region and migrate north in the spring. Along the way, they lay eggs. Over 500 eggs may be laid during an average period of 21 days. Eggs hatch within a few days. Nymphs can survive up to 90 days but usually last only two to three weeks before changing to adults. The entire cycle usually is four to five weeks but this varies considerably depending on host and temperature. There are usually three to four generations per season but fewer as insect migrates further north. Generations may overlap. Appearance of psyllids further north depends on winds starting in June and July.
Population Dynamics
Although most commonly outbreaks occur in Texas, New Mexico, Arizona, Colorado and California, they have been reported as far north as Alberta and Saskatchewan. Potato psyllids overwinter exclusively on winter hosts along the Rio Grande River Valley, on the southern borders of Texas, New Mexico and Arizona. Populations increase from January to May. Peak populations occur at the end of April and early May. They appear north due to migrations of adults. They have appeared consistently in lower and middle High Plains States and in 1998 were reported in South Dakota. Most of the research and available information on this insect pest comes from Colorado and Texas. For a historical perspective, some severe outbreaks in Nebraska, Colorado and Wyoming have been reported.
- Hill, R.E. 1947. An unusual weather sequence accompanying the severe potato psyllid outbreak in 1938 in Nebraska. J Kans Entomol Soc 20:88-92.
- Starr, L.A. 1938. Psyllid yellows unusually severe in Wyoming. Plant Disease Repr 23:2-3.
- Wallis, R.L. 1946. Seasonal occurrence of the potato psyllid in the North Platte Valley. J Econ Entomol 39:689-694.
Potato psyllid can live on or survive on a plethora of hosts. Their main economically important hosts are potato and tomato. However, most members of the Solanaceous family such as pepper, eggplant, and weeds such as nightshades can be hosts. Also, psyllids can survive on many non-Solanaceous weeds such as pigweeds, lambsquarter and bindweed.
Temperature is a critical factor in psyllid outbreaks and range. They do not like very high temperatures, preferring 80 F. Two hours at 100 F is lethal to eggs and nymphs. Temperatures above 90 F will reduce or cease egg laying, hatching and nymph survival. For this reason, psyllids like high altitudes, above 5900 ft. However, their northerly migration seems to be limited by cooler fall temperatures and possibly a reduction in radiance as the autumnal equinox approaches.
Insect Monitoring
In spring, potato psyllids are first found on perennial weedy hosts such as matrimony vine and on greenhouse Solanaceous plants particularly pepper and tomato. Early sprouting potato cull piles also attract the psyllids and these cull piles can then act as a source for potato field infestations. In potato fields, the potato psyllid does not distribute itself uniformly. They typically appear on the edge of the field like Colorado potato beetles, usually the southern rim, and progress toward the center. Yellow pan traps used to monitor green peach aphids will pick up psyllid migrations. Winged black adults can also be picked up using yellow sticky cards hung close to the canopy. Due to the unpredictability of adult migrations and nymph’s toxin inheritance, and toxin effects, no population thresholds have been set for economic levels or treatments.
Damage — Psyllid Yellows

Potato psyllid nymphs attach to the underside of leaves and feed by sucking plant juices, sap, through needle-like mouth parts; this in itself doesn’t cause enough damage to be the concern. But, many nymphs have inherited a gene that causes them to produce a plant poison (phytotoxin) which is injected into the plant in the nymph’s saliva, similar to a mosquito, and drastically reduces or even ceases all plant growth. The degree of damage depends on the amount of toxin, related to insect infestation, and the plant’s growth stage. Variability between cultivars with regard to susceptibility and symptom expression has been suggested, but still unclear, and maybe more related to seasonal maturity type then actual tolerance.
Arslan, A., Bessey, P.M., Matsuda, K. and Oebker, N.F. 1985. Pysiological effect of psyllid on potato. Amer Potato J 62:9-22.
Foliar Symptoms (“Psyllid Yellows”):
Toxic symptoms may appear as quick as four to six days after injection in a heavy infestation but usually it takes two to three weeks. When plant are young, still in vine growth, they will be stunted and chlorotic (yellow from lack of chlorophyll). Internodes will be shorter causing a rosette canopy appearance and nodes will thicken. Occasionally, aerial tubers will appear at nodes.
For field diagnostics, the first and most easily spotted foliar symptom is a distinctive leaf roll (“feathery appearance”) especially in conjunction with yellowing of the upper leaves (see photo). From a distance, this leaf roll looks similar to that symptomatic of black leg, leaf roll virus and Eur. corn borer. But, even without finding the nymphs, the others may be eliminated by looking for the black, slimy stem base associated with black leg, and for the entry hole and fraas associated with the corn borer. Also, unlike leaf roll virus, the leaves are not brittle and do not produce a soft rattle when shaken. The leaf roll is looser with the leaflets are often slightly jagged from irregular growth and the basal part of leaflets are more curled upward. Leaves, especially the younger leaves may have a purple tinge (anthocyanin pigmentation). In some cases, veins may also develop a slight purple tinge in older leaves.
Besides being affected by nymph density, saliva toxicity and feeding duration, several other factors influence expression of foliar symptoms. Higher intensity and longer duration of sunlight will enhance symptom expression. Therefore, the high light intensity and moderate summer temperatures in high mountain valleys as in Colorado are very conducive to psyllid yellows. Symptom expression is also increased in plants in alkaline soils as in the Nebraska Panhandle and by an abundance of irrigation common under center-pivots. Older plants are more tolerant of psyllid infestation and the phytotoxin has a lesser effect since canopy growth is completed but tubers will still be affected unless fully mature.
Tuber Symptoms:

Potato psyllids have a major (big time) impact on yields, the earlier the infestation with regard to tuber growth the greater the yield reduction. Also the severity of yield loss is proportional to the severity of foliar injury. Total yield losses are due to a reduction in tuber size. When infestation is in early bulking, tuber growth is affected more than when infestation is in late bulking. In addition to smaller tubers, there is an increase in the number of tubers and their set is closer to the plant stem, shorter stolons. Furthermore, tubers tend to be mis-shaped and have a rough skin.
Another important tuber characteristic is a loss of dormancy resulting in premature sprouting. Therefore, tuber chaining and internal sprouting are common. Sprouts are spindly, “hair” sprouts, and very weak. These tubers are unacceptable for the following year’s planting. Internal necrosis also may occur but, on the other hand, starch and sugar content are not adversely affected by the phytotoxin.
Reversibility:
Removal of nymphs when the first signs of symptoms seems to allow for recovery of plants. From the University of Arizona, there is a report (Arslan et al., 1985) that, after a planting application of phorate (Thimet), a foliar application of methomyl (Lannate), shortly after infestation at six to seven weeks after emergence, partially reversed the phytotoxicity caused by the psyllids (Figure 1). Note depicted is total yield; marketable yield will be lower as some tubers will still mis-shaped and have sprouting defects.
Control
Chemical — There are many insecticides that will affect potato psyllids. It is best to use a systemic. The main difficulty in treating for them is that the nymphs are on the leaflet’s underside and don’t like to move; therefore, foliar treatments work best when the undersides are exposed. The best foliar applications are low-flying, high-volume aerial spraying and ground spraying using drop nozzles. Common foliar products are Actara, Ambush, Asana, Leverage, Monitor, Phaser, Pounce, Provado and Thiodan. Traditionally, systemic insecticides, Di-Syston and Thimet, were applied at planting but there short residual activity limit their effectiveness. Newer at-planting systemic insecticides, Admire (imidacloprid) and Platinum (thiamethoxam), with their much longer residual, about 13 weeks, have shown to be very effective against psyllids and other insects (Figure 2). Newer still is the use of the active ingredient in these products as seed treatments either as a dust, Tops MZ Gaucho and soon to be released Maxim MZ Cruiser, or as a liquid, Genesis. These treatments seem also to be highly effective against potato psyllids and leafhoppers (Figures 2 and 3).
Biological — There are a number of natural predators that feed on psyllids. These are the convergent lady beetle, minute pirate bug and damsel bug. But, although they are good eaters and will eat any stage of psyllid development, their effect on populations is modest and far less important than weather conditions. There is also a parasitic wasp that will paralyze nymphs and lay their eggs in them. But, field studies show that they are not well synchronized with the psyllid life cycle and therefore do not show much promise.
|
1 Maxim MZ treatments applied at 8 oz/cwt, Tops MZ at 12 oz/cwt, Admire at 1.3 fluid oz/1000 linear feet, and Platinum at 0.55 fluid oz/1000 linear feet.
2 Treatments in columns followed by different letters were significantly different from each other at p=0.05.
Quick Review
Appearance:
- Adult — light green when young, black with white markings when older; 1/10 inch long
- Egg — orange-yellow
- Nymph — pale brown to pale green; tiny, flat, overlapping scales
Life Cycle:
- Overwinters in the Rio Grande River region
- Adults migrate north influenced by wind and temperature
- One to four generations / season depending on geography, temperature and host
Damage:
- Adult — none
- Nymph — injects toxin causing “psyllid yellows” and slows growth of vine and tubers
Management:
- Beneficial predators — lady beetles, pirate bug and damsel bug
- At-planting insecticides — imidacloprid and thiamethoxam
- Foliar treatments
Potato Leafhopper
There are many insects under the umbrella term of “leafhoppers” of which the two that are economically important to potato growers are the aster leafhopper (Macrosteles quadrillineatus) and the potato leafhopper (Empoasca fabae). These are pests throughout the US mid-west and southern Canada. Among entomologists, the leafhopper ranks third as the most important insect pest in North America, after the Colorado potato beetle and green peach aphid. Of the two leafhoppers above, the potato leafhopper has the most effect on yield reduction.
Potato Leafhopper
Potato leafhopper (Empoasca fabae) damages crops through direct feeding on the sap. It is a piercing-sucking insect that causes injury referred to as “hopperburn.” This is characterized by necrosis of leaflets starting at the tips and margins resulting, with progress, in defoliation.
Description
Adult potato leafhoppers are wedge or spindle-shaped, and about an eighth of an inch long. They appear bright lime-green to yellow green with white markings. Wings are transparent green and are folded back when at rest. They have a variable number of white spots on top of their head and along their thorax. Nymphs are similar to adults but are smaller and wingless. Several nymphal stages occur in the generation.
Life Cycle
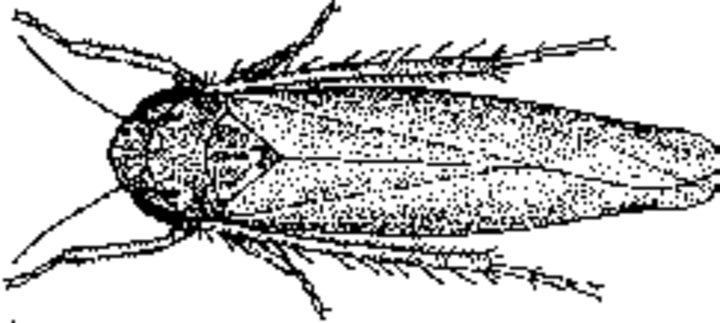

Potato leafhoppers overwinter as adults between southern Louisiana and northern Florida. They fly north in the spring on the winds in the upper atmospheric levels. They are mostly found in Wisconsin and Illinois during the growing season. However, in some years, they have been identified as far west as the Panhandle of Nebraska and as far east as Maine.
Adults live 30-40 days but may live as much as 90 days. Two to three small white eggs are laid per day onto stems or large leaf veins, and 200 eggs per adult life is possible. Potato leafhoppers will lay eggs on potatoes. Eggs hatch in ten days forming the first of several nymphal stages. Nymphal stages are short and within 12 days of hatching the nymphs will become adults. Adults are able to lay eggs six days later. Nymphs move backwards and sideways in a crab-like fashion and cannot move from field to field. There can be one to three overlapping generations per season in the north-central states.
Damage

Potato leafhopper, primarily adults, cause feeding injury to potato plants. They feed on the underside of leaflets. Injury starts with a yellowing along leaflet margins with a slight rolling. This slight injury is soon followed by a gradual browning starting at the leaflet’s tip and margin (hopperburn), and extending basipetally until the leaflet is all dead and desiccated. The browning is due to cellular death or necrosis. Defoliation will occur. The result is a reduction in yield. No effects on tuber quality has been reported by potato leafhopper.
Management
Sustainable Agriculture — Monitoring fields for populations and correctly identifying the leafhopper is essential to good management. There are many leafhoppers that do not damage potato. A threshold for treatment has been established for potato leafhopper as one nymph per 10 leaves. Sample 35 leaves, three to five times in the field.
Chemical Management — Many insecticides will kill leafhoppers. At planting, Admire, Platinum, Thimet and DiSyston are common. Foliar treatments include Actara, Asana, Ambush/Pounce, Baythroid, Dimethoate (formerly Cygon, also used for false chinch bug), Furadan (also used for sand(hill) chafer), Monitor (also used against aphids), Provado, and Thiodan. For gardening, Sevin is recommended
| Seed Treatment | In-Furrow Treatment | Hopperburn % Plants | Yield of US #1 cwt/acre |
| Maxim MZ 10.1D1 | none | 16 A2 | 322 B |
| Maxim MZ 10.1D | Platinum 2SC | 2.7B | 368A |
| Maxim MZ Adage | none | 3.5 B | 389 A |
| Tops MZ 2.5D | none | 11 A | 325 B |
| Tops MZ 2.5D | Admire 2F | 2.1 B | 390 A |
| Tops MZ Gaucho | none | 5.9 B | 377 A |
1 Maxim MZ treatments applied at 8 oz/cwt, Tops MZ at 12 oz/cwt, Admire at 1.3 fluid oz/1000 linear feet, and Platinum at 0.55 fluid oz/1000 linear feet.
2 Treatments in columns followed by different letters were significantly different from each other at p=0.05.
Hosts for potato leafhopper besides potato include edible beans especially lima and snap, alfalfa, and soybeans which is not seriously affected. When alfalfa is harvested, adults may move into potato fields; nymphs die of starvation.
Quick Review / Potato Leafhopper
Appearance:
- Adults — bright green, variable white spots, 1/8 inch long
- Nymph — like adult but smaller and wingless
Life Cycle:
- Overwinters — Louisiana to Florida
- One to three generation/ season in north-central U.S.
- Adults — June to early July
Damage:
- Adult — feeding on sap (sucking-piercing insect)
- Nymph — same
Management:
- Monitor and Identify
- Economic Threshold = 1 nymph/10 leaves
- At-planting Insecticides
- Foliar Insecticide
Aster Leafhopper
There are many insects under the umbrella term of “leafhoppers” of which the two that are economically important to potato growers are the aster leafhopper (Macrosteles quadrillineatus) and the potato leafhopper (Empoasca fabae). These are pests throughout the US mid-west and southern Canada. Among entomologists, the leafhopper ranks third as the most important insect pest in North America, after the Colorado potato beetle and green peach aphid. Of the two leafhoppers above, the potato leafhopper has the most effect on yield reduction.
Aster Leafhopper
Aster leafhopper (Macrosteles quadrillineatus) damages crops not only through direct feeding on the sap but also by being a carrier for a disease “aster yellows.” Aster yellows is caused by a mycoplasma-like organism (MLO) or phytoplasma. These are similar to bacteria but lack a cell wall. They are not air or water-borne but must be transmitted through a carrier or vector.
Description
Adult aster leafhoppers are wedge shaped and thin. They are a tenth to a fifth of an inch long. The bodies are a dull light green and there are six white spots on the top of the head and none on the thorax. The wings are silvery gray and fold back at rest. Like potato leafhoppers, nymphs are similar to adults but are smaller and wingless. Several nymphal stages occur in the generation.
Life Cycle
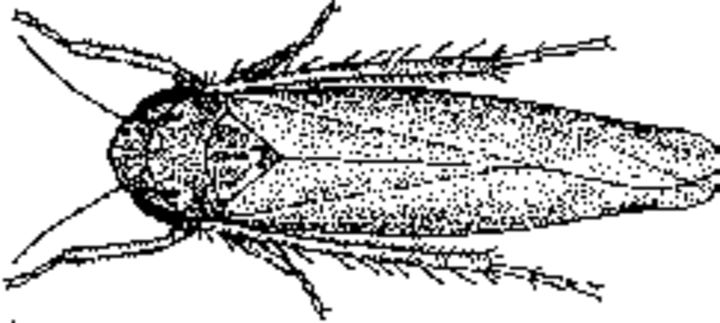
Aster leafhoppers overwinter as adults in northern Texas, Oklahoma and Arkansas. They fly on winds north to Kansas, Nebraska, the Dakotas, Minnesota, and Wisconsin. It overwinters in grassy weeds and small grains. Overwintering as eggs has been reported.
The life cycle is similar to that of the potato leafhopper except that aster leafhoppers do not mate and lay eggs on potatoes. Adults live 30-40 days but may live as much as 90 days and as much as 200 eggs may be laid in an adult’s lifetime. Eggs hatch in ten days forming nymphs which convert to adults within a fortnight.


Damage
Injury by aster leafhopper feeding is minimal and not economically important. The problem rests in that it is a vector (carrier) of the ‘aster yellow mycoplasma-like organism’ (AY-MLO). This MLO causes ‘aster yellows’ also called ‘purple top.’ The MLO is acquired by the aster leafhopper from small grains not from potato. A large aster leafhopper population (severe population pressure) is needed for economic losses. However, yield reduction can occur. Most importantly is that potato tubers from infected plants with produce discolored potato chips and french fries during processing.
Aster Yellow
AY-MLO causes one disease that has many names including purple top and haywire. When as adult aster leafhopper first ingests the MLO, it takes 10 to 21 days for the MLO to replicate sufficiently to be able to be injected into potato plants. How the MLO causes the disease symptoms is not yet known. Flowers are most affected. Petals develop a green pigmentation and there is an abundant production of petals and sepals, both appearing like leaflets. Leaflets will be small, bunched and slightly rolled. A typical character of leaflets is a yellowing or reddening of the leaflet, hence, giving the descriptions of aster yellow and purple top. Aerial tubers and stem swelling around nodes are typically observed. Wilting may occur. Discoloration in potato chips is common. Harvested tubers may or may not be affected for seed. When affected, tubers produce spindly sprouts called hair sprouts. Symptoms vary widely and a laboratory identification is recommended.
Aster leafhopper does not pick up the MLO from potatoes.
Management
Sustainable Agriculture — Monitoring fields for populations and correctly identifying the leafhopper is essential to good management as there are many leafhoppers that do not damage potato. There is no threshold established for aster leafhopper treatment.
Chemical Management — The same insecticides that affect potato leafhopper are effective against aster leafhopper.
The chief important host for aster leafhopper are small grains where they pick up the disease-causing MLO. Potato is not the preferred host of the aster leafhopper.
Quick Review
Appearance:
- Adults — dull green, six white spots, 1/10-1/5 inch long
- Nymph — like adult but smaller and wingless
Life Cycle:
- Overwinters — Texas, Oklahoma and Arkansas
- One to three generation/ season in north-central U.S.
- Adults — June to early July
Damage:
- Adult — injects phytoplasma causing aster yellows feeding on sap (sucking-piercing insect)
- Nymph — feeds off of the sap
Management:
- Monitor and Identify
- Economic Threshold = none established
- At-planting Insecticides
- Foliar Insecticide









